The valency of an atom is determined based on the variety of electrons lost, gained, or shared with one other atom on the time of bond formation. Lewis constructions could be drawn for polyatomic ions as properly. In such situations, the valence electron rely must additionally consider the ion cost. When the polyatomic ion is an anion , one must think about adding electrons to the valence electron depend.
The electron configurations for the primary 20 elements are proven here. I used foam circles I purchased at a craft store for the electron markers. I really have also used candy up to now, but discovered I typically had to replenish the electron supply through the exercise. D- block parts have 1-10 electrons in the d- shell.
Antraniks Smart Core Program
To discover the valence electrons in an atom, identify what group the component is in. For example, Li is in group 1A, so which means it has one valence electron. If the component is in group 2A, then it has two valence electrons. The Group Number represents the variety of valence electrons. The problem is that totally different periodic tables typically use totally different numbering techniques .
- On the other hand, hydrogen acquires the electron configuration of helium and involves a steady state.
- Whereas, valence electrons are associated to elemental characters.
- Here is the list of periodic table of parts with their valence electrons.
- Yes, my curious pals – this is chemical bonding.
So in this sense the $1s$ electrons stay localised to a single atom while the $5p$ electrons are delocalised over each atoms. How can you identify the number of valence electrons from an electron configuration? Remember that our noble gases are throwing a party for every atom and the only requirement is having the appropriate quantity of electron invites.
That is, the iron atom has a total of twenty-six electrons. While the number of valence electrons will increase by one over time, the variety of shells stays fixed. The number of shells around an element’s nucleus is indicated by the period quantity .
At some point the columns might be frivolously shaded and a legend could presumably be added. This works greatest when both all the information is on the table or when discussing properties. A cathode ray tube is a glass tube with two electrodes that are linked to a power cynk elektrony walencyjne source supplying electricity. The anode has a small hole in order that the rays can pass by way of. A phosphor coating on the other end of the tube glows when the cathode rays strike it. Gases which don’t mix with other components to type compounds.
How We Are Able To Determine The Valence Electrons In Phosphorus?
Both valency and valence electrons are applied for any chemical factor. This is a color-coded desk made up of many different squares that lists all of the chemical parts known to humankind. You can usually find these inside the duvet of chemistry textbooks. There can be an excellent interactive desk available online right here. The periodic desk is divided into four blocks by electron configuration.
They are important to the prediction of conduct of an element. Knowing how many valence electrons a component could have allows us to better understand its interactions with different atoms. The ultimate aim of any atom is to realize a full valence shell which would be eight electrons generally. It is more difficult to search out the valence electrons of transition components as they’ve incompletely filled d-subshell and this d-subshell could be very close to the outer s-subshell.
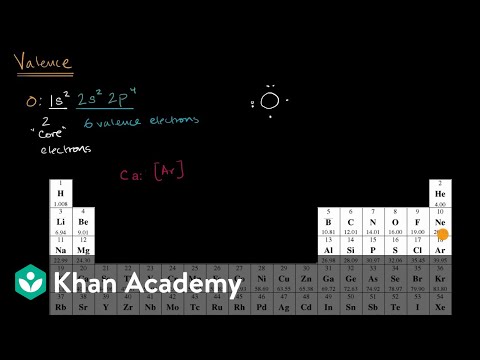
So, let’s revisit our 8 proton/8 electron, attention-seeking atom of the celebration oxygen. Oxygen’s two electrons inside its 2s orbital and 4 electrons inside its 2p orbital are valence electrons. As such, oxygen could be written in core-valence orbital type as 2s22p4. There is a distinction between electrons that are capable of bond – valence electrons – and electrons that don’t – core electrons.
The total number of electrons in the last shell after arranging the electrons of the element known as the valence electron. Depending on the component, the valence electrons could also be paired or unpaired. When only seven electrons are current in the outermost shell of an atom, then the atom is said to possess seven valence electrons. Elements having seven outer-shell electrons are present in group (17) of the Periodic Table.
Atoms with large electronegativities entice the electrons in a bond higher than those that have small electronegativities . The electronegativities of the primary group components are given in the determine beneath. The time period covalent bond is used to explain the bonds in compounds that result from the sharing of one or more pairs of electrons.
